Join us for conversations that inspire, recognize, and encourage innovation and best practices in the education profession.
Available on Apple Podcasts, Spotify, Google Podcasts, and more.
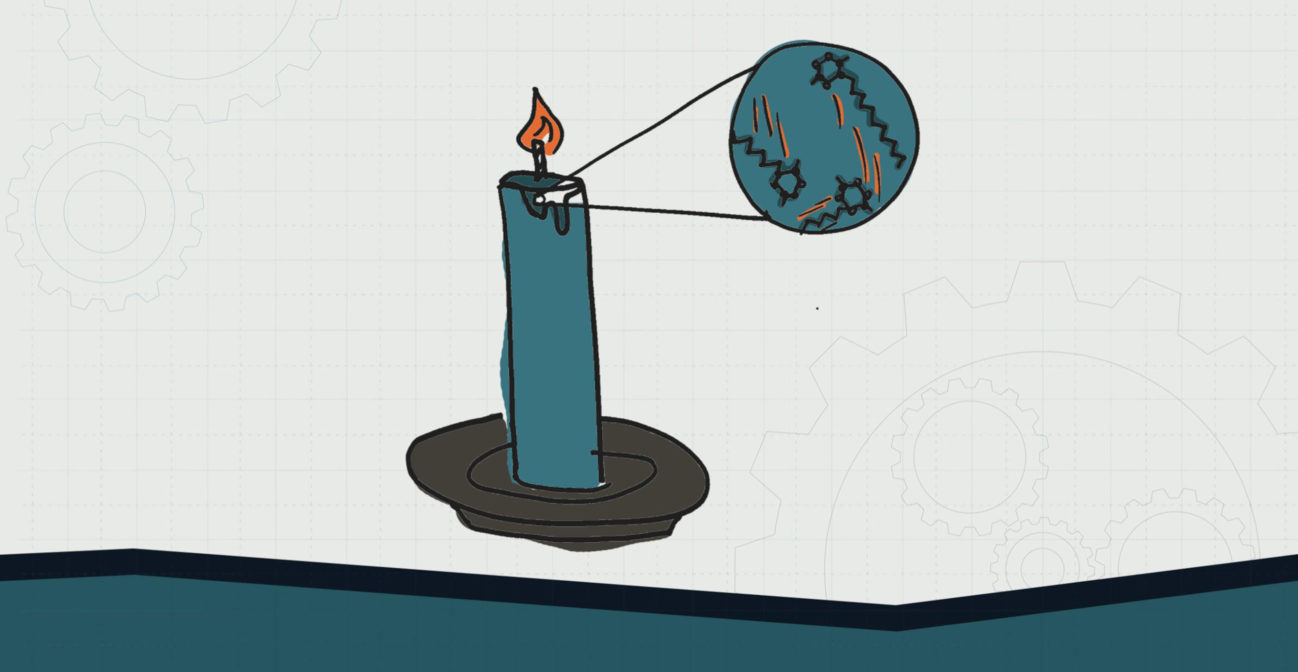
MIDDLE SCHOOL – LEVEL 3
Candle wax drips onto a cake because the flame at the top adds thermal energy to the solid wax. The added thermal energy between the molecules gives the molecules more energy and causes them to move quickly and further apart. This added distance between the molecules causes a weakened force between the molecules and causes a state change. Although we cannot see the change at the molecular level, we see wax change to a liquid phase.
MATERIALS NEEDED:
❏ Lego-style bricks
❏ Cardboard
❏ Any other materials they can upcycle!
❏ Specific materials for activity sequences are in the lesson cycle
DIRECTIONS:
OBJECTIVE: Students will be able to develop a model to explain how thermal energy affects the motion of particles.
ESSENTIAL QUESTION(S):
NGSS CONNECTION:
MS-PS1-4. Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
COMMON CORE CONNECTION:
ELA/Literacy
RST.6-8.7 Integrate quantitative or technical information expressed in words in a text with a version of that information expressed visually (e.g., in a flowchart, diagram, model, graph, or table). (MS-PS1-4)
Mathematics
6.NS.C.5 Understand that positive and negative numbers are used together to describe quantities having opposite directions or values (e.g., temperature above/below zero, elevation above/below sea level, credits/debits, positive/negative electric charge); use positive and negative numbers to represent quantities in real-world contexts, explaining the meaning of 0 in each situation. (MS-PS1-4)
DOK:
Level 3 – Strategic Thinking
Level 4 – Extended Thinking