 |
The concept of atoms was once again introduced to the scientific world by John Dalton in his 1808 book, A New System of Chemical Philosophy. Dalton put forth the concept of all matter being composed of small particles, atoms, which varied in weight and size. An element was a substance that contained only one particular type of atom. The atoms of one element are different from the atoms of any other element. The atoms of elements combine in small whole number ratios to form the many chemical compounds found on earth and in the rest of the universe.
All atoms are composed of a given set of subatomic particles: protons, neutrons, and electrons. These particles have definite arrangements for any given element. The important thing to remember is that the protons, electrons, and neutrons of one element are exactly the same as the protons, electrons, and neutrons of any other element. It is their number and arrangement that make the elements different.
But what are these "protons, electrons, and neutrons"? Let's find out.
|
 |
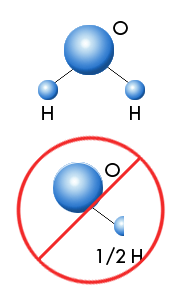 |
| Molecules can only form whole number ratios. There is no such thing as "half an atom." |
|
