 |
About the Groups
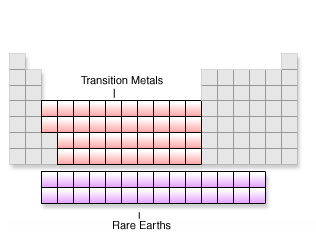
The remaining elements of the periodic table can be lumped into two major divisions, the transition metals (Groups 3-12) and the rare earths (which can be further broken down into the actinides and lanthanides). Each group in the transition metals has complete s and p orbitals with incomplete d orbitals. The elements tend to want the most stable configuration; for example, one electron in each orbital instead of a complete s orbital and four d orbitals with one electron each. This leads to some unique characteristics.
Chemical Properties
The transition elements often act as catalysts in reactions and are often colorful in compounds.
In the "Real" World
Rare earths make the strongest magnets.
Now that you're familiar with the entire Periodic Table, let's see how you do applying some of the lessons you've learned to how elements bond together.


|
 |
|