 |
 When you look at the Periodic Table of the Elements, the energy levels of the atoms correspond to the rows of the table. The two elements in the top row, hydrogen and helium, are filling their first energy level with their final electrons. The eight elements of the second row are filling their second energy level. It is the outermost electrons that determine the chemical properties of the element.
When you look at the Periodic Table of the Elements, the energy levels of the atoms correspond to the rows of the table. The two elements in the top row, hydrogen and helium, are filling their first energy level with their final electrons. The eight elements of the second row are filling their second energy level. It is the outermost electrons that determine the chemical properties of the element.
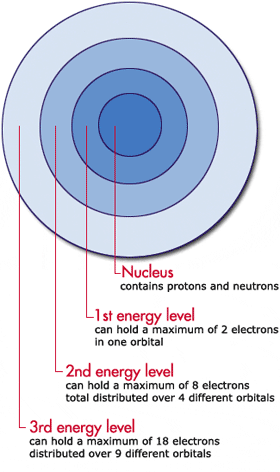
In looking at a diagram of an atom, note that the nucleus is fairly centrally located. The energy levels are built up from the level closest to the nucleus outward. This process of filling in the electrons from the first, lowest energy level to the second, slightly higher energy level to the third, even higher energy level is called filling the electrons in by the Aufbau Principle.
| The Aufbau principle, very simply stated, is: start at the lowest energy level and build up to the higher energy levels only after the lowest are filled. |
But what does it mean to "fill an energy level"? It turns out that electrons exist at energy levels in defined areas known as "orbitals." Let's learn about orbitals now.
|
 |
 When you look at the Periodic Table of the Elements, the energy levels of the atoms correspond to the rows of the table. The two elements in the top row, hydrogen and helium, are filling their first energy level with their final electrons. The eight elements of the second row are filling their second energy level. It is the outermost electrons that determine the chemical properties of the element.
When you look at the Periodic Table of the Elements, the energy levels of the atoms correspond to the rows of the table. The two elements in the top row, hydrogen and helium, are filling their first energy level with their final electrons. The eight elements of the second row are filling their second energy level. It is the outermost electrons that determine the chemical properties of the element.