 |
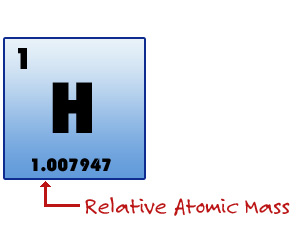
The third item found in each box of any periodic table of the elements is the Relative Mass of the element. By accepted use, the mass of an atom is that of the protons and the neutrons found in the nucleus. Remember that the mass of one electron is so small relative to the mass of a proton or neutron that it would take more than 1,800 electrons to equal the mass of one proton. The number in the box is the mass of one atom compared to one atom of carbon-12. It is also the average of all the masses of all the isotopes of that particular atom, calculated according to the actual abundance of the isotope. We will spend more time on this "weighty" matter in the next chapter.
Finally, some periodic tables may also provide the name of the element, its electron configuration, the various oxidation states the element may exist in, and its electronegativity, boiling point, and state of matter under general conditions. These extras are specific to individual tables and are useful to have but not absolutely necessary.
Play Which One of These Elements Doesn't Belong?
 
|
 |

 |