Join us for conversations that inspire, recognize, and encourage innovation and best practices in the education profession.
Available on Apple Podcasts, Spotify, Google Podcasts, and more.
Unit 10 // Section 1
Industrialized nations rely on vast quantities of readily available energy to power their economies and produce goods and services. As populations increase in developing countries and their citizens demand better standards of living, global energy use will continue to rise, with developing nations accounting for a growing share of total world demand (Fig. 1).
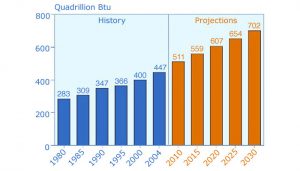
Figure 1. World marketed energy consumption, 1980–2030
See larger image
Source: International Energy Outlook. © 2006. The United States Energy Information Administration.
Today most of the world’s energy is derived from fossil fuels, which are non-renewable resources available only in limited supply. In contrast, many alternative sources of energy, such as wind, solar, and hydropower, are renewable resources because their supplies are refreshed faster than humans consume them. Human society has profited from exploiting energy sources, particularly since energy use became much more efficient during the Industrial Revolution. We are now deeply dependent on reliable, cheap sources of energy. However, it is important to note that energy consumption does not directly improve the human condition. Rather, what matters are the services that we generate using energy.
“Customers don’t want lumps of coal, raw kilowatt-hours, or barrels of sticky black goo. Rather, they want the services that energy provides: hot showers and cold beer, mobility and comfort, spinning shafts and energized microchips, baked bread and smelted aluminum. And they want these ‘end uses’ provided in ways that are secure, reliable, safe, healthful, fair, affordable, durable, flexible, and innovation-friendly.” – Amory B. Lovins and L. Hunter Lovins, “Mobilizing Energy Solutions,” The American Prospect, January 28, 2002
Modern societies also consume vast amounts of material resources, including metals, minerals, stone, chemicals, and fibers. In most cases, these materials are abundant enough that they can be considered either renewable or available in such quantities that we will not soon deplete them. The main concerns associated with material resources, therefore, are generally the costs and environmental impacts of extracting, transporting, and refining them.
Scientists who study energy and material resources seek to understand what types of resources are available and where they can be found and to develop new technologies for locating, extracting, and exploiting them. Discovering new supplies and using more energy and materials is one way to derive more benefits. But we also can use these resources more efficiently, so that we obtain a rising amount of service from a constant level of inputs. Over the longer term, scientific and technological advances may enable societies to substitute new energy sources and material stocks for old ones. This typically happens when new resources perform as well as or better than current options and produce fewer negative impacts, such as pollution or health and safety risks.
But changing from one resource type to another involves more than simply discovering a new mineral deposit or developing new technology. It also means altering the systems that produce, process, and distribute these resources. For example, major commercial energy fuels like coal, natural gas, and uranium are mined, cleaned, processed, refined, and delivered through complex, multi-stage systems that represent billions of dollars in infrastructure investments and complicated logistical interconnections (Fig. 2). Energy facilities typically operate for 30 to 50 years, so they cannot change to different resources or technology mixes overnight. Retiring them prematurely to replace them with something “better” is very costly even if the new plants are not more expensive than the old ones.

Figure 2. Offshore oil drilling platform, Gulf of Mexico
See larger image
Source: © United States Government Printing Office.
Materials Management Service.
This unit describes the main energy sources available or understudies today to meet world demand in the current century. It begins with fuels that have been commercialized and are in use on a large scale, including conventional fossil fuels (coal, oil, and natural gas) and nuclear power. We then consider alternatives such as non-conventional fossil fuels, various renewable energy sources, and hydrogen energy. As we will see, the viability of conventional and alternative energy resources depends largely on developing new technologies that will harness them more efficiently while mitigating their harmful environmental consequences—especially their contributions to air pollution and global climate change.
This unit also surveys major uses of non-fuel mineral (material) resources and their environmental impacts. It concludes with a discussion about using resources efficiently as a way to save money, extend limited supplies, and reduce environmental damages.
Unit 10 // Section 2
Because energy and other mineral resources are so central to our lifestyle in developed countries, they often make news when prices rise and supplies tighten. At these times, national leaders and consumers alike want to know how much of the scarce resource remains, when it will run out, and what alternatives exist. These debates are sometimes cast in stark terms—asking, for example, when the world will run out of oil.
In reality, societies never use up nonrenewable resources completely or exploit the entire flow of renewable resources. Typically the best deposits and sites are found and exploited first, followed by other lower-quality sources as demand rises. As demand grows and a resource becomes scarce, its price rises. This reduces demand and gives explorers incentive to develop sources that are lower-quality and/or more expensive to exploit and to improve technologies for locating, extracting, and processing the resource.
Rising prices also spur the development of substitutes that were uneconomic when the original resource was cheap. For example, as discussed later in this unit, high oil prices are driving significant investments today into fuel production from plant sources (Fig. 3). In the words of Sheikh Zaki Yamani, a former oil minister of Saudi Arabia, “The Stone Age did not end for lack of stone, and the Oil Age will end long before the world runs out of oil” (footnote 1). The race is between finding new supplies and exploiting them more efficiently on one hand and declining resource abundance and/or quality on the other.

Figure 3. Pump offering bio-based fuels, Santa Fe, New Mexico
See larger image
Source: © Bensinger, Charles and Renewable Energy Partners of New Mexico.
The concepts of stocks and lows are important in thinking about resource supplies. A stock is the amount of material in a certain deposit or reservoir—for example, the total quantity of oil in a field that can be recovered with today’s technology. Flow refers to the rate at which new material is added to the stock (inflow) or removed from the stock (outflow). The net flow rate (inflow minus outflow) determines whether the stock grows, shrinks, or remains constant.
Non-renewable resources are limited by the size of their stock, but energy developers consider stocks on several levels. For example, total U.S. copper resources include all known copper deposits and those that are estimated or believed to exist, even if they cannot be economically found or extracted with today’s technology. Reserves are the subset of this supply whose location is known or very likely based on geological evidence and that can be extracted profitably with current technology at current prices. A larger fraction, often referred to as unrecoverable or ultimately recoverable reserves, will require technical advances to locate and develop economically.
These categories are imprecise and shift as exploration and technology breakthroughs enable us to recover supplies that once were out of reach. Figure 4 shows current estimates of how many trillion cubic feet of natural gas the United States has in each of these categories—including sources such as methane hydrates (discussed further below) that cannot be exploited today but could become an important source in coming decades.
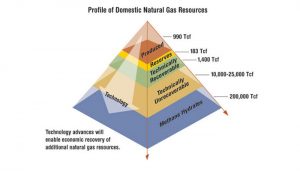
Figure 4. Profile of domestic natural gas resources.
See larger image
Source: © United States Department of Energy.
In contrast, the use of renewable resources is limited by their flow rate, which can be divided into the total flow and exploitable flow—the portion that can be practically exploited with current technology. The fraction of the total flow that is exploitable depends on the abundance of sites where the resource is sufficiently concentrated and close enough to the point of end-use to be harnessed economically—a question that naturally depends in part on the state of the technology available for doing so. For example, the United States has good wind resources in the Great Plains states, but many of the windiest regions are far from major electricity demand centers, so the cost of building long-distance transmission lines affects decisions about where wind farms are built.
Unit 10 // Section 3
Oil, natural gas, and coal, the traditional fossil fuels that have powered modern societies since the Industrial Revolution, remain the dominant world energy sources today. These fuels account for roughly 80 percent of world energy use and 86 percent of that of the United States, where the remainder comes from nuclear power and renewable fuels (Fig. 5).
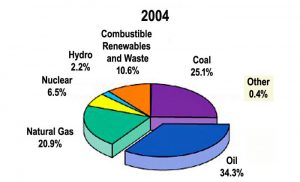
Figure 5. World energy use by fuel source
See larger image
Source: Key World Energy Statistics, p.6. © Office for Economic Co-operation and Development/International Energy Agency (2006).
Coal, the first fossil fuel exploited by humans for energy on a large scale, is a carbonaceous rock formed from buried plants in ancient forests or swamps. These plant materials are initially converted to peat—a loose, brown, organically rich soil that itself is an important energy resource in some areas. As more rock layers press down on the buried deposits, geothermal energy heats the peat and reduces its oxygen and hydrogen content, converting it to coal (Fig. 6). As materials go through this process, known as thermal maturation, their energy content by weight increases.
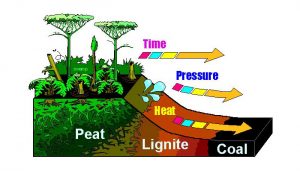
Figure 6. Coal formation
See larger image
Source: © Steve Greb, Kentucky Geological Survey
Coal comes in several grades that reflect its thermal maturity and energy content:
Coal is extracted in both subsurface and surface strip mining operations. These processes have significant but different environmental impacts. Underground mining has a relatively low immediate impact at the surface but can cause ground subsidence when mineshafts collapse. Coal dust and methane gas (which is commonly found along with coal) raise significant risks of explosions. Worldwide, several thousand miners on average die each year in coal mining-related accidents.
In contrast, the impacts of strip mining—removing soils and overburden to extract shallow coal deposits—are highly visible at the surface. Strip mining operations generally leave permanent scars on the landscape. In its most extreme form, mountaintop removal, the land is clear-cut and leveled with explosives to expose coal seams, with most of the removed overburden dumped into neighboring valleys (Fig. 7).

Figure 7. Mountaintop removal site
See larger image
Source:© Vivian Stockman/www.ohvec.org. Flyover courtesy SouthWings.
Beyond the mine, coal produces significant amounts of atmospheric pollution and greenhouse gas emissions when it is burned. Coal combustion generates sulfate and nitrogen emissions that contribute to acid deposition, regional haze, and smog. It also produces mercury, which accumulates in the fatty tissues of animals and fish and can harm humans who consume certain species. (For more on these issues, see Unit 11, “Atmospheric Pollution.”) And coal is the most carbon-intensive of all fossil fuels, so it produces a disproportionate share of total greenhouse gas emissions from energy use. On average, coal contains roughly 30 percent more carbon per unit of energy than crude oil and 75 percent more than natural gas (footnote 2). (For more discussion of GHG emissions from energy consumption, see Unit 12, “Earth’s Changing Climate.”)
A variety of technologies exist to make coal cleaner to burn, including filtration systems that reduce particulate emissions, scrubbing systems to reduce hazardous sulfur and nitrogen emissions, and methods for removing mercury. Moreover, coal can be turned into a form of syngas (synthetic natural gas), which can be burned with smaller environmental impacts. Many of these technologies are proven and some are in use at modern power generation facilities. It is generally very costly to retrofit older power plants with these capabilities, however. Technologies that could capture a large part of the carbon dioxide from coal-burning power plants, for subsequent storage away from the atmosphere, are under intensive development but are certain to be expensive.
Unit 10 // Section 4
Oil and natural gas are formed primarily when marine organisms die and settle to the seafloor in anaerobic environments where they cannot rapidly decompose, they are buried by sediments and heated by Earth’s geothermal gradient (the rate at which temperatures increase moving inward from the surface toward the planet’s core). This process breaks down complex organic molecules into a viscous gel called kerogen, which evolves with further heating into hydrocarbons—organic compounds consisting of carbon and hydrogen atoms. Petroleum and natural gas, and the products that we derive from them, such as gasoline and diesel fuel, are composed of hydrocarbon molecules of various sizes and shapes.
Most oil and gas forms in source rocks that represent ancient sea beds buried and heated to a certain temperature range, which corresponds to a depth range below the surface often called the oil or gas window. The oil window is generally between 1 and 6 kilometers deep, depending on the type of kerogen present and the geothermal gradient, so it is rare to produce oil from deeper levels. Gas can be produced and retained at much greater depths, such that some important gas deposits reside at depths greater than 10 km.
Oil and gas migrate out of source rocks into porous and permeable rocks called reservoirs and collect in traps that are often formed by faults or folded rocks. Reservoirs must be overlain by impermeable rocks called cap rocks or seals. The combination of source rock, reservoir, trap, and caprock is called a hydrocarbon system—the essential geologic elements that must be in place to yield a large oil or gas field (Fig. 8).
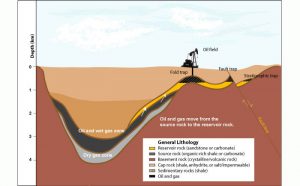
Figure 8. Cross-section of a hydrocarbon system
See larger image.
Developers tap these deposits by drilling wells into oil and gas reservoirs. In many cases, natural pressures drive the hydrocarbons to the surface. For certain heavy oils, or in fields where pressure has been depleted by production, the oil must be pumped to the surface or driven from below by injecting water, natural gas, CO2, or steam into the reservoir. In many parts of the world, oil and gas exploration is pushing the frontiers of technology, with developers drilling wells more than seven miles below the surface, in deep water, or horizontally through reservoir rocks.
Refineries distill crude oil to produce a wide range of fuels, lubricants, and industrial chemicals. On average, about half of a standard barrel of oil (42 gallons) is converted to gasoline. Refined petroleum also yields kerosene, jet fuel, diesel fuel, home heating oil, and lubricants in varying proportions, depending on the original type of crude oil and the refining process (Fig. 9). Natural gas may also require processing to remove undesirable gases such as hydrogen sulfide and other impurities. In some cases, this process can yield useful byproducts, such as sulfur, which is sold and used to generate fertilizer and for a wide range of other industrial purposes.
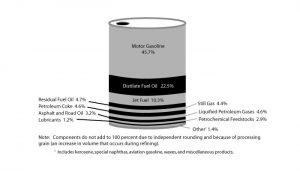
Figure 9. Products from a barrel of crude oil
See larger image
Source: © adapted from the United States Coast Guard original.
Oil and gas drilling can have adverse environmental impacts, from surface disturbance for construction of drilling pads and access roads to contamination of aquifers with drilling muds and fluids. Offshore drilling can cause spills and leaks that pollute ocean waters, either as a result of industrial accidents or through storm damage to drilling rigs. Transporting oil and gas from wells to processors to users also requires large infrastructures and creates environmental risks. Oil is shipped worldwide by pipelines and tankers, both of which are subject to spills. Most natural gas is currently transported via pipeline, but tanker shipment of liquefied natural gas (LNG) that has been chilled to -260°F represents a growing segment of the world market. LNG is re-gasified at receiving terminals and delivered by pipelines to end-users.
Oil produces somewhat lower levels of CO2, sulfur dioxide, nitrogen oxide, and mercury emissions than coal when it is burned, but still contributes significantly to acid rain, photochemical smog, and global climate change. Natural gas combustion emits lower amounts of nitrogen oxide and CO2 and virtually no sulfur dioxide or mercury. (For more details, see Unit 11, “Atmospheric Pollution,” and Unit 12, “Earth’s Changing Climate.”)
Modern practices of drilling for and producing oil and gas attempt to minimize adverse environmental impacts. For example, co-produced waters are now generally re-injected or cleaned before disposal, and enhanced safety systems and procedures have made drilling and production accidents rare. Oil spills from tankers still pose a serious environmental hazard, but national governments have agreed on steps such as eliminating old tankers in favor of double-hulled designs by 2015 in an effort to further reduce these risks.
Nevertheless, because the United States has exploited many of its prime oil and gas reserves, exploration on land is now moving into environmentally sensitive regions, such as public lands that hold fossil fuel deposits but also are home to rare and endangered species. As a result, the environmental impacts of oil and gas exploration have become highly controversial in many parts of the western United States.
Unit 10 // Section 5
Vast quantities of oil and gas are held in deposits where they cannot be produced by conventional drilling. When the world price of oil is high, it becomes more profitable to find ways of exploiting these unconventional fossil fuel resources. For example:

Figure 10. Athabasca tar sands, Alberta, Canada
See larger image
Source: © Suncor Energy Inc.
Unit 10 // Section 6
Nuclear energy, which generates about 17 percent of world electricity supplies (roughly 6 percent of total energy consumption), is produced by enhancing the radioactive decay of naturally fissile materials—elements whose atoms can be split by thermal (slow) neutrons, releasing energy. About 0.7 percent of natural uranium consists of the isotope uranium-235, which is fissile and is the most widely-used fuel in standard nuclear reactors. The remainder is the more stable uranium-238.
To exploit this energy source, companies mine uranium ore and, by a process called uranium enrichment, increase the concentration of U-235 to about 4 percent. Enriched uranium is formed into fuel rods or pellets, which are placed inside a nuclear reactor and bombarded by neutrons. This process causes U-235 atoms to split into two or more smaller atoms, called daughter products and releases large amounts of energy. This process also releases excess neutrons, which split other U-235 atoms, causing a nuclear fission chain reaction.
Operators control the rate of fission using control rods and moderators that absorb excess neutrons and by adjusting the reactor temperature, which affects the reaction rate. Energy generated in the reactor heats water, steam, or some other fluid, which is pumped from the reactor and used to produce steam that drives electric turbines (Fig. 11).
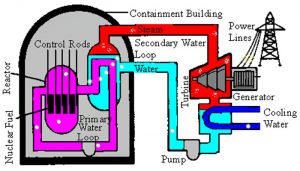
Figure 11 Pressurized-water reactor
See larger image
Source: Courtesy of the University of Wisconsin-Madison.
Nuclear power is a well-established method of electric power generation. Uranium is abundant, and several countries are making substantial investments in new nuclear power reactors. The United States has more than 100 licensed commercial nuclear power reactors, but no new reactor has been ordered since 1978, although interest has revived in recent years.
Major obstacles to the expansion of nuclear power worldwide include concerns about safety and high capital costs compared to other energy sources. Nuclear accidents at the Three Mile Island plant in Harrisburg, Pennsylvania, in 1979 and the Chernobyl reactor in Ukraine in 1986 convinced many people that nuclear power was unsafe. Chernobyl caused more than 30 deaths in the days immediately following the accident (from acute radiation exposure), and widespread exposure to radioactivity from the accident over a large part of the Northern hemisphere may ultimately lead to tens of thousands of deaths from cancer for decades. Although both accidents were largely results of human errors, and modern facilities have much more substantial safety procedures, these events demonstrated that nuclear accidents were possible.
Spent nuclear fuels remain highly radioactive for thousands of years, and finding appropriate sites to store radioactive waste is a highly contentious issue in virtually every nuclear nation. The United States is struggling to build and license a national repository at Yucca Mountain, Nevada, after decades of study (Fig. 12), but concerns persist about whether the site’s complex geology can isolate nuclear waste from the environment until its radioactivity decays to background levels. This failure has forced many nuclear power stations to store their spent fuel onsite for years longer than owners planned and has undercut public support for new nuclear reactors in the United States.

Figure 12. Main tunnel shaft, Yucca Mountain repository site
See larger image
Courtesy Daniel Mayer, 2002. Wikimedia Commons, GNU General Public License.
In addition to these environmental impacts, nuclear power also raises security concerns because it produces two types of fissile material that can be used in nuclear weapons. First, as noted above, uranium fuel for commercial power reactors is enriched to a concentration of about 4 percent U-235. Although this low-enriched uranium is not usable for weapons, the same facilities can often enrich uranium to 90 percent U-235 or higher, and this highly enriched uranium is the easiest material from which to make a nuclear weapon.
Second, when nuclear fuel is irradiated in a reactor, a portion is converted to plutonium, which is also fissile and, given somewhat greater skill on the part of weapon-makers than is needed for a uranium bomb, can also serve in the weapon role. The process of plutonium production is enhanced in certain modern reactor designs called fast breeder systems, which use plutonium as an additional source of nuclear fuel and are now in use in several nuclear nations. Plutonium fuel cycles pose increased proliferation risks because plutonium can be stolen or diverted while it is being handled in bulk quantities during fuel processing.
Unit 10 // Section 7
Biomass energy sources are all around us. They include many types of plants and plant-derived material, such as crops and wastes; trees, forestry wastes, and mill residues; animal wastes; and municipal solid waste. About 11 percent of world primary energy today is produced from biomass, but this comes mostly from low-technology applications in developing countries, such as burning crop waste, wood, or dung for home heating and cooking.
While these fuels might seem like low-impact energy sources, biomass burning in primitive stoves is very inefficient and generates large quantities of pollutants such as fine particulates, sulfur and nitrogen oxides, and carbon monoxide, which are especially harmful when released in poorly ventilated houses. Indoor air pollution is a serious health risk and causes many respiratory illnesses in developing countries (for more details, see Unit 6, “Risk, Exposure, and Health”). The inefficient combustion of biomass fuels in primitive stoves also means that much of their energy content is wasted. Programs to help users switch fuels or install more efficient stoves are among the cost-effective options to reduce health impacts from using traditional biofuels (Fig. 13).

Figure 13. A ceramic cook stove saves fuel in Myanmar
See larger image
Source: © G. Bizzarri, Food, and Agriculture Organization of the United Nations.
Advanced biomass technologies that use organic material cleanly and efficiently offer much greater opportunities. One of the fastest-growing biomass applications today is the production of transportation fuels from plant sources. Notably:
Both ethanol and biodiesel are viable sources of renewable energy that can reduce our dependence on conventional fossil fuels and reduce harmful emissions. However, growing biofuel crops—especially corn for ethanol—requires major quantities of fossil fuel to manufacture fertilizer, run farm machines, and ship the fuel to market, so these biofuels do not always offer significant net energy savings over gasoline and diesel fuel. Growing corn is also water-intensive and removes significant levels of nutrients from the soil (hence its high need for fertilizer). Also, relying too heavily on these sources could mean diverting crops from the food supply at some point to produce energy.
Analysts generally agree that, at best, corn ethanol offers modest energy savings over gasoline, and that the real promise lies in making ethanol from cellulosic (woody) plants such as switchgrass, willows, and poplars (footnote 3). Some of these plants, notably switchgrass, also sequester large amounts of carbon, restore nutrients to the soil, and can be used to stabilize land threatened by erosion (Fig. 14). Cellulosic plant tissue is tough and must be broken down before it can be fermented, but contains substantially more energy per unit than carbohydrates such as corn. Current research focuses on developing quick and efficient methods of breaking down cellulosic plant tissue for fermentation into fuel (footnote 4). Many experts believe that cellulosic ethanol will become a significant energy source in the United States in the next one to two decades.
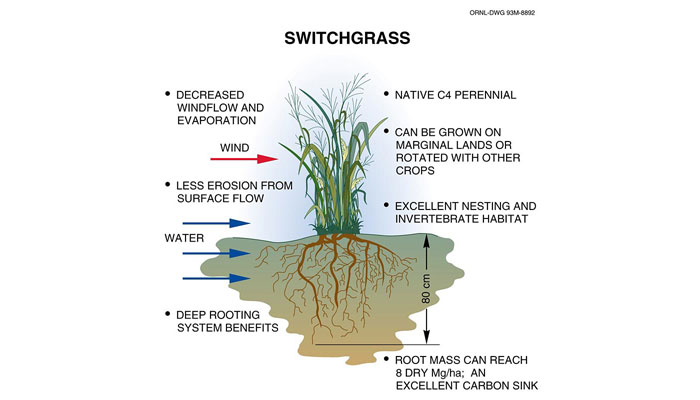
Figure 14. Switchgrass
See larger image
Biomass can also be used to generate electricity. Some U.S. power plants either run completely on biomass fuels or co-fire them with coal to reduce emissions. In most cases, biomass fuel is burned directly to boil water and turn steam turbines, but some advanced plants convert biomass fuels to gas by heating them in a low- or zero-oxygen environment. The resulting gas burns more efficiently than solid wood waste or plant material, thus extracting more energy from the fuel with fewer pollutants (footnote 5). Many industrial facilities, especially in the pulp and paper industry, produce significant quantities of electric power using residual biomass fuels such as wood pulp that are generated from their production facilities.
Human and animal wastes can also produce electricity. On farms, devices called anaerobic digesters use microbes to break down manure into organic solids and biogas, which typically contains about 60 percent methane and 40 percent CO2. The methane can be burned to generate electricity and reduce greenhouse gas emissions. Similarly, many large landfills collect the biogas that is generated by decomposition of buried organic waste and burn it to generate electricity.
One of the most important environmental benefits of using biofuels is that biomass energy is carbon-neutral—that is, using it does not increase long-term greenhouse gas levels in the atmosphere. Biomass fuels such as timber release carbon when they are burned, but this carbon was sequestered from the atmosphere when the original trees grew and would have been released when they died and decayed, so using biofuels simply completes the natural carbon cycle. In contrast, burning coal or oil releases carbon that was previously sequestered underground for millions of years and would have stayed there if it were not mined for energy, so it represents a net transfer of carbon from terrestrial sinks to the atmosphere. (For more on the carbon cycle, see Unit 2, “Atmosphere.”) Biomass energy thus does not contribute to global climate change unless it is harvested more quickly than it regenerates—for example when large forest areas are clear-cut.
Unit 10 // Section 8
Hydropower and ocean energy systems indirectly tap the Earth’s solar energy flux, which drives the cycling of water between Earth’s surface and the atmosphere and heats the upper layer of the oceans (for more details, see Unit 8, “Water Resources”). By damming rivers for hydropower or placing turbines in zones of the ocean areas with significant tides and currents, we can use the power of flowing water to generate electricity. Today hydropower generates about 17 percent of world electricity supplies (the same as nuclear energy, although hydropower is credited with only a third as much primary energy as nuclear because it does not generate two units of heat for every unit of electricity). Ocean power systems are still at an experimental stage.
Like biomass, hydropower is an established renewable energy technology that is widely used in many parts of the world. The basic technology is simple: falling water flows through pipes, called penstocks, then turns turbine blades to spin a generator and produce electricity (Fig. 15). When excess power is available from the grid, some hydropower stations pump water up to storage reservoirs and hold it in reserve, then release it to generate power when demand rises.
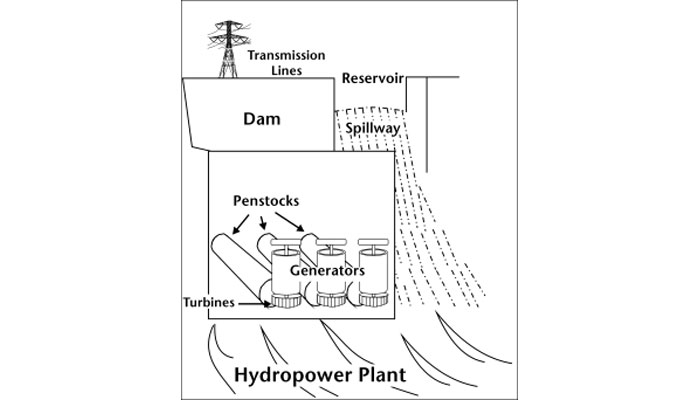
Figure 15. Hydropower system
See larger image
Hydropower generates electricity without producing significant air pollution, except for emissions from building and maintaining dams. Large hydropower dams can also serve other purposes: for example, the reservoirs that develop where rivers are dammed can provide drinking water supplies, and many are used for fishing and boating. Some hydropower reservoirs, especially in dry regions like Africa, have become important habitats for birds.
In recent years, however, critics have drawn attention to hydropower’s negative environmental impacts. A report issued in 2000 by an independent international commission cataloged ways in which large dams can harm ecosystems, such as:
The report also noted that while hydropower does not generate greenhouse gas emissions as the water spins electric turbines, reservoirs emit CO2 and/or methane from rotting submerged vegetation and carbon inflows from the catchment area. Calculating how much a specific dam contributes to climate change depends on many factors, including whether the flooded land was previously a carbon source or sink and what land-use changes result from building the dam and displacing people from the flooded area. On balance, however, it appears that warm, shallow tropical dams emit more GHGs than deep, cold dams at higher latitudes (footnote 6).
In spite of these negative aspects, hydropower is an attractive alternative to fossil fuels for many countries with good resources. In addition to their low pollutant emissions, hydropower plants provide dispatchable power: their output can be raised or lowered quickly to meet fluctuating levels of demand. Other renewable sources, such as wind and solar energy, produce energy intermittently when the wind blows or the sun shines, so they are not as responsive to daily market conditions.
At best, however, world hydropower capacity can be expanded only by a factor of 2 or 3 because a limited number of good sites remain available for development, mainly in Africa, Asia, and Latin America. In the United States, it is estimated that more than half of the hydropower generating capacity is already tapped, and most of the remaining potential dam sites would adversely affect sensitive environments. China’s Three Gorges Dam, the largest hydroelectric project in the world, is scheduled to enter operation in 2009 (Fig. 16). The project will provide 18,000 megawatts of electricity-generating capacity—an important asset for China, which relies heavily on coal to meet its fast-growing energy needs. However, it has been criticized for flooding many rural valleys and displacing more than 1.5 million people.

Figure 16. Aerial view of the Three Gorges Dam, China
See larger image
Ocean energy occurs in the form of tides, waves, currents, and heat. Tidal energy resources are modest on a global basis, and tapping them involves building major dams on inlets and estuaries that are prized for other purposes, so few tidal energy facilities have been developed. Harnessing waves and currents on a significant scale will involve designing turbine structures that are large, inexpensive, and can operate for long periods under the physical stresses and corrosive forces of ocean environments. For the most part, such systems are at the research stage today.
The largest but most experimental form of ocean energy is ocean thermal energy conversion, which taps heat stored in the ocean to generate electricity. This process runs warm surface seawater through several different types of systems that use the water’s stored heat to turn a turbine, then cools the resulting steam or vapor with cold deep-seawater (footnote 7). Making this conversion work affordably on a large scale is technologically very difficult because it requires large structures and physical challenges associated with working in the ocean environment. It works most effectively in regions where there are large temperature differences between surface and deeper waters, mainly in the tropics. If ocean thermal energy conversion can be commercialized at some point, however, it could become an enormous new energy source.
Unit 10 // Section 9
Geothermal power systems tap our planet’s natural radioactive energy and the fact that temperature and pressure inside Earth increase with depth. Earth’s geothermal gradient is steeper in some regions than others, generally because of volcanic activity or large natural deposits of naturally radioactive material in granitic rocks. Energy companies can drill a mile or more to tap underground reserves of steam and hot water, much in the same way as they drill for oil and natural gas.
Early geothermal plants used steam pumped directly from underground. Today, however, most geothermal power plants pump water down into wells, use subsurface heat to warm it, and return it to the surface to form steam, which drives electric turbines to generate electricity. Geothermal power has been an established technology since the early 20th century and is economically viable in geologically suitable sites, such as in the Geysers field in northern California or in Iceland, which produces most of its energy in this way.
Geothermal energy is considered a renewable resource because it draws from the essentially unlimited heat in the Earth’s interior. Where resources are good, it produces reliable power with virtually no atmospheric pollutants or greenhouse gas emissions. In the United States, most of the best geothermal resources are located west of the Mississippi River (Fig. 17). It has proven difficult to extend this technology to areas with great demand for electricity, such as the eastern United States and much of Europe, because the local geology does not provide sufficiently high subsurface temperatures. Thus, geothermal power is a minor component of energy supply in most parts of the world today. In the future, it may prove viable in areas of tremendous geothermal potential to use excess geothermal energy to produce more transportable forms of energy—for example, by extracting hydrogen from seawater.
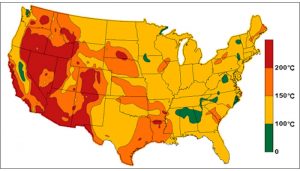
Figure 17. U.S. geothermal resources (estimated temperature at 6 kilometers depth)
See larger image
Source: © 2006. United States Department of Energy. Energy Efficiency and Renewable Energy.
Unit 10 // Section 10
Wind power also indirectly taps Earth’s solar energy flux, which causes differences in atmospheric temperatures and pressures that drive winds (for more details, see Unit 2, “Atmosphere”). Wind turbines directly harness wind power and convert it into electricity. Today wind power generates less than 0.5 percent of the world’s electricity supply, but it is growing rapidly and has several times the growth potential of hydropower. Wind power scales with the area swept by the blades (proportional to the square of the blade diameter) and the cube of the wind velocity. The best locations for wind farms thus are areas with consistently high winds where large turbines can be situated. Turbines can be located both on land and in shallow waters offshore.
Advances in wind turbine technology, such as larger and more durable towers, are making wind power more efficient and economically competitive relative to fossil-fuel-fired and nuclear-generated electricity. Several European countries have made major investments in wind energy, both for domestic power production and as a high-technology export. Most notably, Denmark generates 20 percent of its electricity from wind and is a major supplier of wind turbines around the world (Fig. 18).

Figure 18. Offshore wind turbines near the southwest coast of Denmark
See larger image
Source: © Sandia National Laboratory.
Wind turbines generate electricity without producing air pollutants or greenhouse gases. Concerns about the environmental impacts of wind energy centers on finding appropriate sites for wind farms. Some critics argue that wind towers mar natural settings, such as ridgelines and coastal areas, while others worry that turbines blades will kill large numbers of birds and bats. Some early wind power installations, such as the turbines in California’s Altamont Pass, had significant impacts on birds, but the industry has learned from these cases. Today, wildlife issues can usually be managed with careful siting processes and thorough environmental reviews. Replacing any significant fraction of fossil fuel consumption with wind power will require widespread siting of turbines, so resolving these concerns is a key step for the expansion of wind energy.
Unit 10 // Section 11
Direct solar power systems typically generate electricity in one of two ways. Photovoltaic (PV) cells, the type commonly seen on homes and commercial buildings, use semiconducting materials such as silicon to produce electricity from sunlight: when light hits the cells, the material produces free electrons that migrate across the cell, creating an electric current. Alternatively, large-scale solar concentrating systems focus sunlight with mirrors to heat a liquid that boils water, creating steam to turn a turbine. Solar energy can also be used for residential water heating (replacing fossil fuels or electricity) by circulating household water or a heat-carrying fluid through roof-mounted solar collection systems.
Concentrating solar power is best suited for power plants in areas with strong sunlight and clear skies, like the southwestern United States, while PV and solar hot water systems can be used in a wide range of climates and latitudes. PV cells are used widely as power supplies in electronic consumer goods such as hand-held calculators. However, because PV technologies for consumer applications have a maximum efficiency of about 15 percent, large expanses of PV cells are required to generate significant amounts of electricity. It would take more than 25 square kilometers of standard photovoltaic cells to generate the same amount of electric power as a large coal-fired power plant.
Residential PV systems are now available at major home-supply stores in several states that offer financial incentives to promote home solar power, including California and New Jersey. Some developers are integrating them into energy-saving home designs (Fig. 19). Making solar energy cost-competitive on a large scale will require further gains in efficiency and reductions in the cost of manufacturing PV cells.

Figure 19. Solar-powered housing complex, Watsonville, California
See larger image
Source: © Dan Coyro.
Unit 10 // Section 12
As economies develop and mature, they tend to follow an energy path that moves from high carbon/low hydrogen fuels at early stages to fuels with higher hydrogen and lower carbon contents. Typically, nations use wood as their main primary fuel at a pre-industrial stage, shift to coal during industrialization, and then transition to oil and natural gas as their economies mature. This progression takes place because each new fuel is cleaner-burning and easier to distribute and store (once an infrastructure has been built to handle it) than its predecessor. The United States and western Europe are beginning to plan for perhaps the next stage on the decarbonization path—hydrogen—but this transition will require several decades to design and deploy systems for producing, transporting, and using hydrogen fuel.
Although it is sometimes called a “fuel of the future,” hydrogen is more accurately described an energy carrier. Like electricity, pure hydrogen does not occur naturally in quantities worth harnessing to meet human energy needs: the main naturally-occurring stocks of hydrogen are tied up in chemical compounds, most importantly water molecules (H2O) and hydrocarbons such as coal (approximately CH), oil (approximately CH2), and methane (CH4). Stripping hydrogen from hydrocarbon fuels or obtaining it by splitting water using electricity or heat is not technically difficult, but in any of these approaches, more electricity or primary-fuel energy is used than the resulting hydrogen contains.
The benefit of paying this energy price to get hydrogen comes in the form of hydrogen’s portability, storability, amenability to high-efficiency application not only in combustion engines but in fuel cells (discussed below), and low emissions. One application currently under research is the use of hydrogen fuel cells to power cars (Fig. 20).

Figure 20. View under the hood of a fuel cell car
See larger image
Source: © National Renewable Energy Lab.
Today, the oil and chemical industries worldwide use about 50 million tons of hydrogen each year, most of it extracted from natural gas and coal. Deriving hydrogen from fossil fuels emits CO2, so scaling the process up would increase greenhouse gas emissions unless the associated carbon were captured and stored (for more details on carbon capture and sequestration, see Unit 13, “Looking Forward: Our Global Experiment”).
Hydrogen can be burned directly to generate energy or used in devices such as fuel cells that combine hydrogen and oxygen to produce electricity, with water as a byproduct (Fig. 21).
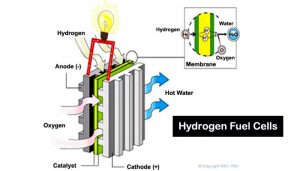
Figure 21. Hydrogen fuel cell
See larger image
Source: © 2001. Rocky Mountain Institute/www.rmi.org.
Here is how the basic process works:
Existing fuel cell technologies can convert as much as 70 percent of hydrogen’s energy content to electricity. None of the basic designs in use today are cheap and technically simple enough yet for mass production, although they have been used for applications such as producing power on manned space missions.
Over the past several years, politicians and scientists have endorsed the idea of converting to a hydrogen economy. This transition poses many challenges. In addition to producing hydrogen economically and commercializing fuel cells, it takes seven times as much hydrogen on a volume basis to produce the same amount of energy in a gallon of gasoline. Therefore, adopting hydrogen as a fuel will mean building new energy storage and distribution systems nationwide. The devices that convert hydrogen to energy services—cars, heating systems, and consumer goods—will also have to be converted. Most expert assessments of the timing for a hydrogen economy project that such systems will not start to be deployed on a large scale until 2020 or later, and that making a full transition from fossil fuels to hydrogen in the United States would take until approximately 2050 or later.
Unit 10 // Section 13
Fuels are not the only resources that we mine from the earth. Modern society could not exist without numerous materials such as metals, rocks, and minerals that serve as raw ingredients for buildings, technologies, and industrial processes. In large part, industrialization is a process of refining and employing these materials in ever more sophisticated ways and of substituting machine power (which relies on metals and other advanced components) for human labor.
Metals are used widely in construction, manufacture, and consumer goods and are important material resources for industrial societies. Steel, which is a blend of iron and other compounds, is king of the metals, accounting for more than 90 percent of global metal production. Many other metals, such as chromium and nickel, are mined primarily for blending with iron to form steels with desirable characteristics, such as corrosion resistance.
Metals are extracted from ores[/tooltip—naturally-occurring rocks and minerals that hold large deposits of high-quality, readily harvested metals. Ores form through many geological processes, including volcanism, underground water flow, and rainfall that alters soils. For example, iron ore deposits generally form when water mobilizes reduced iron (Fe2+) and the iron oxidizes, forming Fe3+, which is insoluble in water and precipitates. This process deposited vast quantities of iron ore on ancient seafloors, yielding what are called banded iron formations. Iron ores also form when underground water percolates through porous rocks such as sandstone, forming ironstones.
The first step in making steel is to extract iron from ore in a process called smelting, a chemical reduction process whose basic steps are several thousand years old. Pulverized ore (taconite) is heated by burning coke (a coal derivative) together with limestone to remove waste materials (Fig. 22). The process yields a mixture of iron and carbon, a residue called “slag,” and some emissions. Steel, which is stronger than iron and has other superior qualities such as hardness, corrosion resistance, and electrical conductivity, is made by processing the molten iron-carbon mixture to remove excess carbon while adding other elements (for example, nickel, vanadium, or molybdenum) to obtain the desired properties.

Figure 22. Iron smelting, Carrie furnaces, Rankin, Pennsylvania, 1952
See larger image
Perhaps the second most important metal today is aluminum, which is light, tough, and corrosion-resistant and has high electrical conductivity. Aluminum metal is used today in many manufactured goods, including cars and planes as well as smaller consumer goods.
The primary ore of aluminum is bauxite, which forms when high volumes of rainwater move through soils. Typically the water dissolves and removes elements such as sodium, potassium, and calcium, leaving altered soils called laterites that contain significant amounts of highly insoluble metals such as aluminum. Laterites are widespread in tropical environments. To mine aluminum, developers strip off the topsoil and overburden to extract ore, which can require drilling and blasting. Much like coal strip mining, aluminum mining uproots vegetation, displaces wildlife, and pollutes area lakes and rivers.
Aluminum ore is smelted through a complicated process that involves extracting aluminum oxide, then passing high-voltage electricity through it to free the aluminum metal. The process is very energy-intensive: aluminum manufacturers are some of the largest industrial consumers of electricity worldwide and many are located in regions like the Pacific Northwest, where regional electricity prices are relatively low. Aluminum production also generates large quantities of greenhouse gases, although many major companies have formed partnerships with the government to reduce these emissions.
Many other metals are important for specialized industrial and manufacturing purposes. For example, copper is used primarily as a conductor of electricity, while titanium is used as a lightweight metal alloy and for white paint pigments. Mining and smelting operations for many metals are similar to the processes involved in making iron. In some ores, such as copper, the metal is bound with sulfur, so mining and smelting these metals produces sulfuric acid and environmental impacts similar to those of acid rain (for more details, see Unit 11, “Atmospheric Pollution”).
Sulfide mines and smelting operations often leave major environmental scars. For example, the Berkeley Pit, a former open-pit copper mine in Butte, Montana, is one of the most polluted water bodies in the United States (Fig. 23). The pit contains over 30 million gallons of water with a pH value of 2.5 (highly acidic) that has drained from mine tunnels and shafts feeding into it and is laced with arsenic and heavy metals including aluminum, cadmium, copper, iron, lead, zinc, and sulfate. In 1995, 342 migrating snow geese died when they mistook the pit water for a safe resting place. Six companies have agreed to pay for cleaning up the pit at an estimated cost of $110 million (footnote 8).

Figure 23. Berkeley Pit, Montana
See larger image
Unit 10 // Section 14
A wide variety of other material resources are extracted from geological deposits, including stone building materials, fertilizers, and industrial chemicals. Building stones, which are chosen for a combination of durability and aesthetic appeal, include marble, granite, limestone, slates, and sandstones. Aggregates such as crushed stone, gravel, and sand are also widely used for construction and to manufacture concrete and other treated rock products. Rock products are used directly as insulators, abrasives, and paint pigments and in many other applications.
Before the industrial era, farmers used manure and bone meal as fertilizers, but manufacturing fertilizer has become a major industry. As discussed in Unit 4, “Ecosystems,” and Unit 7, “Agriculture,” the primary elements of fertilizers are nitrogen, phosphorus, and potassium. Today potassium and phosphates are mined from ancient marine beds, while nitrogen is extracted from the air by burning natural gas to form ammonia (NH3).
Rocks and minerals also are used as feedstocks for a wide variety of industrial chemicals such as sulfuric acid, flame retardants, wood preservatives, glass, ceramics, paper coatings, and battery and computer chip components. Ore deposits for the raw materials to manufacture most of these products are generally plentiful, so the economics of extracting, processing, and transporting feedstocks generally dictates which materials are developed.
Unit 10 // Section 15
Thirty years of fluctuating world energy prices since the Arab oil embargos of the 1970s, as well as growing concerns about the environmental impacts of producing and using energy, have made it increasingly clear that “more of the same” is not a sufficient basis for national energy planning. Many of the best world oil and gas resources are being fully exploited, and resource depletion is making the remaining supplies more expensive. Some oil experts contend that world oil production will peak in the near future, although others strongly disagree and argue that technology improvements will continue to make new reserves exploitable. We can expand energy supplies by using alternative sources and technologies that usually have lower environmental impacts, but this typically costs more until new sources are fully commercialized. The same is true for some of our important material resources.
When resources become scarce, we can benefit from learning to get more services from a given flow of energy or stock of material. For example, we can design cars that are stronger but weigh less than today’s models and get more miles per gallon, buildings that require less energy per square foot to heat and cool than current structures, refrigerators that require less power per cubic foot of interior volume, lights that deliver more illumination for each kilowatt-hour of electricity they consume, and manufacturing processes that use less energy to make a given array of products.
Industrialized and developing countries alike have greatly increased their energy efficiency in recent decades. The United States doubled the overall energy efficiency of its economy between 1970 and 2005. That means that this country was extracting twice as much real gross domestic product from each unit of energy flowing through the economy in 2005 as it did 35 years earlier. There are many areas in which we can continue and accelerate these trends by using energy and materials even more efficiently.
Investments into research and development of end-use efficiency challenges often pay for themselves many times over in resulting savings. Figure 24 shows representative estimated savings from energy efficiency upgrades in a home located in an average U.S. climate and equipped with standard appliances.
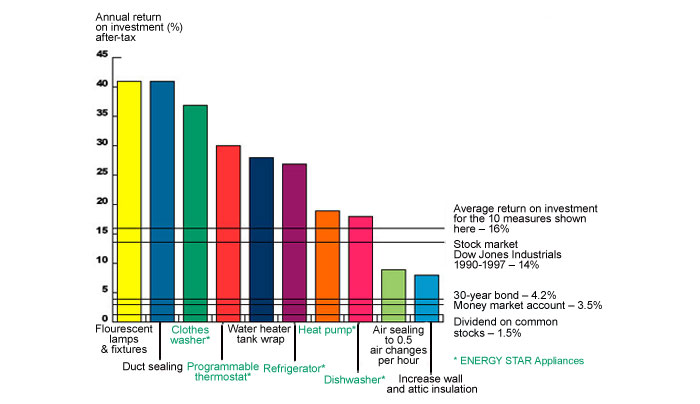
Figure 24. Profitability of energy efficiency upgrades
See larger image
When a refrigerator saves a kilowatt-hour of electricity or an efficient car saves a liter of fuel, that energy is available for use elsewhere in the economy. This means that improving end-use efficiency is like finding a new supply of energy. It is often cheaper, faster, and cleaner to reap gains from end-use efficiency (sometimes referred to as “negawatts,” to connote energy that does not have to be produced) than to expand energy supply through exploration and drilling. Similarly, investing in recycling programs, better product design, and longer product lifetimes, we can reduce our need for newly-mined minerals.
Unit 10 // Section 16
“Fuel Cells: Collecting History with the Worldwide Web,” Smithsonian Institution, http://americanhistory.si.edu/fuelcells/intro.htm. An online project documenting the development of fuel cells, with descriptions and diagrams of many fuel cell technologies.
National Research Council and National Academy of Engineering, The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs (Washington, DC: National Academies Press, 2001). A broad assessment of issues involved in making a 50-year transition to a hydrogen economy in the United States.
Michelle Nijhuis, “Selling the Wind,” Audubon, September/October 2006. Expanding wind power is a balancing act between energy concerns and ecosystem protection.