Join us for conversations that inspire, recognize, and encourage innovation and best practices in the education profession.
Available on Apple Podcasts, Spotify, Google Podcasts, and more.
Look for the following topics in the video, indicated by the ![]() onscreen icon, and click below to learn more.
onscreen icon, and click below to learn more.
 Why do Snowflakes Have Six Sides? |
 Helium vs. Hot Air Balloons |
When temperature and humidity conditions are conducive, individual water molecules from small droplets of liquid water in the atmosphere, particularly in clouds, can condense slowly to form solid water or ice.
 A snowflake is built up molecule by molecule. Each time a growing snowflake moves past water droplets, several molecules of water are added to it. As we mentioned in the video for Session 6, in order to explain this behavior, we must refine our model of the water “particle” from a simple sphere to a small “V” shape, as shown in the following picture:
A snowflake is built up molecule by molecule. Each time a growing snowflake moves past water droplets, several molecules of water are added to it. As we mentioned in the video for Session 6, in order to explain this behavior, we must refine our model of the water “particle” from a simple sphere to a small “V” shape, as shown in the following picture:
The central atom is an oxygen atom, which has hydrogen atoms bound to it on either side. The angle between the two arms of the molecule is 104.5 degrees, first determined around 1930 by x-ray diffraction techniques.
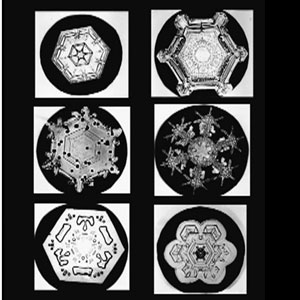
Several snowflake formations.
The oxygen atom has a particularly strong attraction to the electron clouds of the two hydrogen atoms and pulls them closer. This leaves the two hydrogen ends more positively charged, and the center of the “V” more negatively charged. When other water molecules “brush up” against this growing snowflake, strong forces between the negatively charged and positively charged parts of different particles cause them to join together in a very specific three-dimensional pattern with a six-sided symmetry. Each water molecule that joins the snowflake reflects this pattern until eventually we can see its macroscopic six-sided shape.
If snowflakes are built from a particular pattern, why aren’t all snowflakes identical?
As a snowflake moves up and down in the atmosphere, slight changes in temperature and humidity cause the exact pattern to change as it is built. The overall shape of each flake, however, remains six-sided.
In this video session, we stated that helium balloons rise in air for the same reason that a piece of pinewood rises in water — the buoyant force upward is greater than the weight downward. We can also state this as “the weight of the watery (or airy) ghost is more than the weight of the object.”
If an object has an average density (mass divided by volume displaced) that is less than that of the fluid in which it is placed, it will rise. If the average density is greater than the density of the fluid in which it is placed, it will sink.
In the case of the wood, water molecules bumping into the sides of the wood provide the pressure that causes it to rise. With the balloon, moving molecules of nitrogen, oxygen, and argon collide with the outside of the balloon to provide pressure and thus the buoyant force that causes it to rise.
But how can balloons be less dense than air? Let’s take a closer look at two types of balloons that rise in air.
 Recall that the particles of a gas are, on average, very far apart and collide less frequently than do particles of liquids or solids. Consider two balloons that contain gas and have equal volumes. If one balloon is filled with air and the other is filled with helium, the balloons will still contain the same number of particles. Why? Since the spaces between particles are quite big (about 1000 times the diameter of a single molecule), the size of the individual particles could be larger or smaller by a factor of two or three and not change the total volume of gas. Therefore, a liter of air contains the same number of particles as a liter of helium.
Recall that the particles of a gas are, on average, very far apart and collide less frequently than do particles of liquids or solids. Consider two balloons that contain gas and have equal volumes. If one balloon is filled with air and the other is filled with helium, the balloons will still contain the same number of particles. Why? Since the spaces between particles are quite big (about 1000 times the diameter of a single molecule), the size of the individual particles could be larger or smaller by a factor of two or three and not change the total volume of gas. Therefore, a liter of air contains the same number of particles as a liter of helium.
Air is made mostly of the molecules oxygen and nitrogen. The most common type of isotope for O_2 (oxygen) [link to isotopes ACL in session 5] contains eight protons and eight neutrons. The most common type of isotope for N_2 (nitrogen) contains seven protons and seven neutrons. Helium is made of single atoms of helium, the most common isotope of which contains two protons and two neutrons. Thus, the mass of one particle of helium (a single atom) is about one eighth the mass of one particle of oxygen (a molecule with two atoms of oxygen). If the same volume of these two gases contains the same number of particles but the mass of each helium particle is much less than the mass of a particle of air, the density of the helium will be less than that of air, thereby causing helium to rise in air.
In summary, there are the same number of particles in a helium balloon and an air balloon of equal volume, but each particle of helium has a smaller mass.
Let’s compare a balloon with cool air and one with hot air to help explain the differences in density.
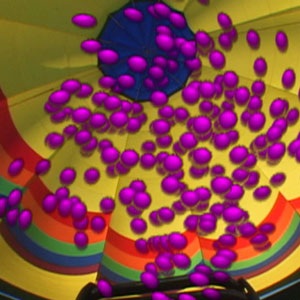
Particles in a hot air balloon.
As we’ll see in Session 7, as an object becomes hotter, its particles move faster, collide more often, and therefore have larger spaces between them. In this case, the balloon with the hot air will have larger spaces between its particles and, as a result, a smaller number of particles in the same volume.
The particles of air in each balloon individually weigh the same but, since there are fewer particles in the hot air balloon, there is less mass in that air than in cooler, more dense air. Since the two balloons have the same volume, the density of the hot air is less than that of the cool air and the hot air balloon will float in air!